Education: Degrees of success
Life-science PhD graduates who wish to leave academia often find rewarding careers in the laboratories of biotechnology and pharmaceutical companies. But some find that the lab isn't enough. Researchers who choose to move beyond the bench to the upper levels of the company often decide to add three more letters to their CV: MBA.
Investing time and money in another degree may seem an unappealing prospect for many PhD holders, but that's the reality of the competitive job market: sometimes you have to go beyond the usual training to get the job. An MBA (master of business administration) can open up career possibilities for a biotechnology or drug-development researcher and help them to stand out from the crowd. Those who decide to take the plunge face key questions: how and when to pursue an MBA (see 'When to go for an MBA'), and where to go from there. Many who have travelled this path say that the extra effort to get the degree has paid off by taking their career to the next level.
An MBA can help industrial researchers to move to a higher position and earn more. Jane Rhodes, now a manager for new high-tech initiatives at Biogen, a biotechnology company in Cambridge, Massachusetts, had spent ten years at the company working on drugs for neurological disorders such as Parkinson's and Alzheimer's disease. She felt hemmed in by the lab, but she realized that she didn't have the business or management skills to move up the company ladder. “I came through the British education system, which is very focused,” she says. “I wanted to learn more about the business side of biotech.”
To fill that gap, Rhodes embarked on a two-year MBA programme at Babson College in Wellesley, Massachusetts. Specifically designed for mid-career professionals, the programme took up to 30 hours a week, a big commitment for a researcher who already had a full-time job and a family. The programme would have cost her about US$75,000, but Biogen paid the bulk of the tuition bill, a sign of how much the company values the degree and the person.
Rhodes used her MBA to get her job at Biogen overseeing new company initiatives, a position that would have been off-limits without the extra training in the business side of science. “I can now move to multiple different positions across the company,” she says. “The combination of PhD and MBA is very valuable.” She enjoys thinking beyond the confines of research — and that's only one benefit of her revitalized career. “Without an MBA,” she says, “I don't know if my salary would be anywhere close to what it is now.”
An MBA could give industrial researchers the insight they need to help turn a business around. Looking back, Oréda Boussadia wishes that she'd had that insight in addition to her research skills. She was one of only a few people in the world who knew how to create a certain type of transgenic mouse, thanks to her PhD and postdoctoral training in France and Germany. But she knew nothing about turning mice into profits, which was a problem at the small French biotech company that she joined after her postdoc. “We had very good results, but we had trouble making sales,” she says. The company failed within a year, forcing Boussadia to quickly ponder her next step. “I really wanted to continue in biotech, but I had to refine my management skills,” she says. “I knew how to design a research project, not how to develop a company.”
Boussadia jump-started her career by enrolling in the MBA programme at the Institut Français de Gestion in Nantes, France. Like other MBA schemes, it focused on the practical aspects of business: product development, market analysis, pricing and return on investment, using real-life examples as learning tools. Degree in hand, she soon got a job managing the production and sales of transgenic mice at a branch of Charles River Laboratories in Lyon, France. After holding that job for five years, she is now the European head of business development and strategy for EpiVax, a biotech company in Lyon. She's happy with the course of her career. “I enjoyed research, but it wasn't enough,” she says. “I wanted to be a decision maker.”
New horizons
Armed with an MBA, many can leave the lab without leaving science. As a postdoc, Kyle Rasbach investigated potential therapies for muscular dystrophy at the Dana–Farber Cancer Institute in Boston, Massachusetts. But thanks to the MBA that he'd pursued along with his PhD, he was snapped up after his postdoc for a job studying investment opportunities at investment management firm T. Rowe Price in Baltimore, Maryland. Much of his remit involves evaluating the research taking place at drug companies, from the giants of the business to small start-ups. His lab background helps him to spot blockbuster drugs in the making. “Sixty to seventy per cent of my job is science-based,” he says. “You can't do this job and be excellent at it without a PhD or an MD.”
That's also true for Moritz Fischer, director of international marketing for Fresenius Medical Care in Hessen, Germany. After earning his medical degree at the Ludwig Maximilian University of Munich in Germany, he realized that he did not want a career as a physician or clinician. He took a job at Fresenius as a lower-level marketing manager, but soon recognized that he could go much further with advanced business skills. So he pursued an MBA at Danube University Krems in Austria. The company covered his tuition, which he estimates would have cost him at least €20,000 ($22,500). It was a reasonable investment for the company, he says, because he has made money for them. “They were able to capitalize on my training,” he says.
" I enjoyed research, but it wasn't enough. I wanted to be a decision maker. "
Success stories of researchers with MBAs in biotech and drug development have caught the attention of early-career researchers who are still plotting their careers. Jeffrey Zahratka, a postdoc at the Cleveland Clinic in Ohio, says that he could see himself working at a biotech firm, perhaps one that makes implantable devices to treat neurological disorders. “I could act as a go-between for the research side and the business side,” he says. He still has to weigh up the pros and cons of another degree, but he thinks that he could bring a lot of value to a company. “People with a research background have a lot of tenacity,” he says. “They are battle-tested.”
If he decides to go down the MBA route, he won't be alone. But for now, PhD–MBA remains a relatively rare combination — that factor alone can help a person to stand out and move forward. It's a matter of degree.
CÉRÉMONIE DE CLÔTURE DE L'ANNÉE UNIVERSITAIRE 2015/2016
Fidèle à ses traditions, l’université des Frères Mentouri Constantine organisera la cérémonie de clôture de l’année universitaire 2015/ 2016 et ce le 30 juin 2016 à l’auditorium Mohammed Saddik Ben yahia à partir de 09.30.
Pour cette occasion, le Rectorat récompensera les étudiants classés majors de promotions dans les différents parcours de formations (en Licence, en master et en doctorat) dispensés par les facultés et les instituts de l’Université.
A cet effet, toute la communauté universitaire est cordialement invitée.
International Conference on Integrated Environmental Management for Sustainable Development
|
|
Appel à candidatures pour les formations ouvertes et à distance diplômantes L3, M1 ou M2 (FOAD) 2016-2017
L'AUF propose 88 formations diplômantes pour la promotion 2016-2017 sur sa plate-forme deFormations ouvertes et à distance (FOAD) :
- 7 Diplômes Universitaires en Médecine (DU) ;
- 11 Licences (L3) ;
- 20 Masters 1 ;
- 50 Masters 2.
- Droit, Économie et Gestion ;
- Éducation et formation ;
- Sciences de l’ingénieur ;
- Médecine ........................ Lire la suite
Changement de paradigme dans l'enseignement tertiaire
Amélioration de la qualité et de la gouvernance en faveur de la compétitivité et de l'employabilité du 30 Mai 2016 au 1 Juin2016
La 7ème Edition du Salon de l’Emploi le 1 & 2 Juin 2016
L’Université des Frères Mentouri Constantine avec son Centre des Carrières organise les 1 & 2 Juin 2016, la 7ème Edition du Salon de l’Emploi qui sera placée cette année sous le slogan :
« un Entreprenariat Université/entreprise pour l’insertion professionnelle des diplômés»
A cet effet, les professionnels des secteurs socio-économiques, présenteront aux étudiants des idées de montage d’entreprise, les démarches de candidature à un poste de travail, les profils requis et l’évolution du marché du travail.
Un programme très riche est au RDV lors de ces deux journées tels que : La présentation du projet européen COFFEE qui concerne la co-construction de licences professionnalisantes avec implication du secteur socio-économique, des conférences, des expositions,…
Les Conférences auront lieu à la salle de conférence de la faculté des Lettres et Langues, l’Exposition des stands au Hall du Bloc des Lettres de l’Université des Frères Mentouri Constantine.
Entomology: Nabokov's scientific artistry
Vladimir Lukhtanov delights in a treatise on the luminary's contribution to biology.
Vladimir Nabokov's influence on Russian and English literature and language is assured. Many people also know of the novelist's lifelong passion for butterflies. But his notable contributions to the science of lepidopterology and to general biology are only beginning to be widely known.
Nabokov was no amateur entomologist. He served for six years as curator of the butterfly collection at Harvard University's Museum of Comparative Zoology in Cambridge, Massachusetts, and published a dozen papers on taxonomy — the description and classification of organisms — that remain important. His observations on butterfly morphology have stimulated breakthrough research in evolutionary biology. Several of his original biogeographic hypotheses have been confirmed in the past few years. Fine Lines, a collection edited by Stephen Blackwell and Kurt Johnson, explains the importance of Nabokov's scientific work and traces its influence on his novels.
Reproduced by permission of the V. Nabokov Estate/the Henry A. & Albert W. Berg Collection, New York Public Lib.
One of Vladimir Nabokov's drawings of the undersurfaces of butterfly wings.
The book begins with 154 of Nabokov's black-and-white and colour drawings of butterflies' fine anatomical structures. Most represent the European, Asian and American species of the 'blues' of the tribe Polyommatini, Nabokov's favourite group. Ten essays follow, by prominent researchers including evolutionary biologist James Mallet, current Harvard butterfly curator Naomi Pierce and lepidopterist Robert Pyle, explaining the interplay of science and art in Nabokov's writings. Fine Lines clearly demonstrates the significant impact that science had on Nabokov's evolution as a writer.
The decision to open the book with the drawings is a masterstroke. They illustrate one of the most important aspects of Nabokov's creativity — his tremendous attention to details, described with scrupulous precision. In his novels, he seamlessly marshals minutiae — impressions, passing fancies, ideas — to create a universe strongly rooted in observation. The particular or apparently trivial was, for him, always worth probing. In his entomological studies, he analysed fine, nearly invisible, dots on the wings of New and Old World butterflies to hint at what may have happened on Earth millions of years ago. With no palaeontological data, Nabokov speculated that North and then South America were populated by five waves of butterflies migrating from Asia ( Psyche 52, 1–61; 1945) — a picture confirmed by DNA analysis almost 70 years on ( et al. Proc. R. Soc. B 278, 2737–2744; 2011).
This pointillism is harder than it seems. Piling up millions of elements can easily end in chaos; to create a picture, one needs to understand the nature of these elements and to be able to choose between them. The core of scientific drawing differs greatly from photography in focusing on the heart of the matter and avoiding unnecessary details. This is important for science, and no less for art. Both have the same central goal — to reveal an unknown or invisible essence of things. That is one of the main points of Fine Lines.
Yet science and art diverge in their communication. In science, the ability to convey the idea properly and simply is a matter of special talent, but almost everyone can learn to do it. Not so in art. Nabokov's drawings are scientifically perfect, but also staggeringly fine aesthetically. They show how the merging of content and form in art conveys ideas wonderfully. However, even the most wonderful idea becomes banal if artistry is lacking.
The personal, artistic and scientific aspects of Nabokov's life were tightly intertwined. As one of the book's essayists, science writer Dorion Sagan, concludes, nature and art were a continuum for him: “the distinct but equally necessary paths of art and science seem to scale opposite sides of the same majestic mountainscape”.
Nabokov's fiction is permeated by science, as Fine Lines amply reveals. He was a master in the use of motif and symbols. In his novel Lolita (Olympia, 1955), for instance, the town Lepingville is named after 'lepping', butterfly hunters' slang for chasing butterflies, and Elphinstone after Elphinstonia, a subgenus in the white butterfly genus Euchloe. The fictional play-within-the-novel, The Enchanted Hunters, is built almost entirely on symbols associated with butterflies. Diana, its protagonist, is both the virgin goddess of hunting and a butterfly species (Speyeria diana). In his essay, Nabokov scholar Brian Boyd reveals that Edusa Gold, who directs the play, is an echo of Colias edusa, an old but preocccupied name for the clouded yellow (now Colias croceus). I can add that her sister Electra Gold was named after Colias electra, an unavailable name for the African clouded yellow, now Colias electo. That these names are effectively hidden — no longer in use, but buried in lists of unavailable scientific epithets — chimes with the secrecy in this controversial novel.
I prepared this review at the Nabokov House Museum in St Petersburg, Russia. While there, I discovered in the Nabokov family's copy of An Illustrated Natural History of British Butterflies and Moths by Edward Newman (William Glaisher, 1870) that Nabokov had, as a child, coloured in the black-and-white image of the clouded yellow with remarkable accuracy. As zoologist Victor Fet describes in Fine Lines, Nabokov's childhood concentration on butterfly collecting and drawing effectively provided very specific training in memory and paying attention, as well as that focus on minute detail.
Few have so beautifully and meaningfully meshed serious scientific endeavour with artistic brilliance, visual and verbal. Fine Lines helps us to understand the phenomenon of creativity, without which neither good science nor true art can exist.
Journée d'Orientation et de Promotion de l'Investissement Agricole
Le 23 Mai 2016
Campus 500 Places
Programme
|
8h30mn |
Accueil des participants |
|
Cérémonie d’Ouverture |
|
|
10h30 |
Visite des stands |
|
12h30 |
Allocution de Monsieur le Recteur de l’Université des Frères Mentouri Constantine 1 Allocution du Directeur des Services Agricoles de Constantine. Allocution de Monsieur le Wali de la Wilaya de Constantine. Allocution de la Cérémonie Officielle par Monsieur le Ministre de l’Agriculture et du Développement Rural et de la Pêche |
|
|
Pause Café |
|
|
|
|
12h45 |
« Programme de la pisciculture intégrée à l’agriculture » par Dr MAKHZAR Meriem, Chef d’antenne Pêche Constantine |
|
13h00 |
« Les fromages traditionnels Algériens opportunités de développement pour les PME » par AISSAOUI-ZITOUN O. , BENYAHIA F.A. , BOUGHELLOUT H. , ADOUI F. , SIAR H. et ZIDOUNE M.N. INATAA, Equipe : Transformation et Élaboration de Produits Agroalimentaires, Laboratoire de Nutrition et Technologie alimentaire (L.N.T.A.) |
|
13h15 |
« Les nouvelles procédures de partenariat, sur les terres privées de l’état » par Mr GHANEM Hamza, Responsable contentieux, ONTA-Constantine |
|
13h30 |
« Opportunités d’investissement dans l’industrie agro-alimentaire » par DIM Constantine |
|
13h45 |
« Les différents types de crédits liés à l’investissement » par le Directeur de la BADR -GRE- |
|
14h00 |
« Levures oléagineuses : la biotechnologie au service de l’agriculture » par Naila Doria BOUCHEDJA, Abdelghani BOUDJELLAL, Franck DELVIGNE, Sabine DANTHINE INATAA-UFMC, Equipe MAQUAV- Laboratoire BIOQUAL Université Liège-Gembloux/AgroBioTech, unité de sciences des aliments de formulation, Belgique. |
|
14h15 |
« Présentation de l’unité pain sur table et son impact sur la région est-entreprise citoyenne » par Mr ZEROUKI |
|
14h30 |
« La recherche au service du développement du secteur agro-alimentaire » BOUGHALLOUT H. INATAA, Equipe : Transformation et Élaboration de Produits Agroalimentaires, Laboratoire de Nutrition et Technologie alimentaire (L.N.T.A.). |
|
14h45 |
« Valorisation de la paille par l’urée » par Dr MEZIANE Toufi, ISV-Batna |
|
15h00 |
Débat et recommandations |
|
15h30 |
Clôture |
|
|
|
CRISPR: gene editing is just the beginning
Molecular biologists are riding a wave of new technologies made possible by CRISPR.
Whenever a paper about CRISPR–Cas9 hits the press, the staff at Addgene quickly find out. The non-profit company is where study authors often deposit molecular tools that they used in their work, and where other scientists immediately turn to get them. It is also where other scientists immediately turn to get their hands on these reagents. “We get calls within minutes of a hot paper publishing,” says Joanne Kamens, executive director of the company in Cambridge, Massachusetts.
LISTEN
Heidi Ledford talks Noah Baker through the cutting edge of the CRISPR technique
Addgene's phones have been ringing a lot since early 2013, when researchers first reported1, 2, 3 that they had used the CRISPR–Cas9 system to slice the genome in human cells at sites of their choosing. “It was all hands on deck,” Kamens says. Since then, molecular biologists have rushed to adopt the technique, which can be used to alter the genome of almost any organism with unprecedented ease and finesse. Addgene has sent 60,000 CRISPR-related molecular tools — about 17% of its total shipments — to researchers in 83 countries, and the company's CRISPR-related pages were viewed more than one million times in 2015.
Much of the conversation about CRISPR–Cas9 has revolved around its potential for treating disease or editing the genes of human embryos, but researchers say that the real revolution right now is in the lab. What CRISPR offers, and biologists desire, is specificity: the ability to target and study particular DNA sequences in the vast expanse of a genome. And editing DNA is just one trick that it can be used for. Scientists are hacking the tools so that they can send proteins to precise DNA targets to toggle genes on or off, and even engineer entire biological circuits — with the long-term goal of understanding cellular systems and disease.
“For the humble molecular biologist, it's really an extraordinarily powerful way to understand how the genome works,” says Daniel Bauer, a haematologist at the Boston Children's Hospital in Massachusetts. “It's really opened the number of questions you can address,” adds Peggy Farnham, a molecular biologist at the University of Southern California, Los Angeles. “It's just so fun.”
Here, Nature examines five ways in which CRISPR–Cas9 is changing how biologists can tinker with cells.
Nature special: CRISPR
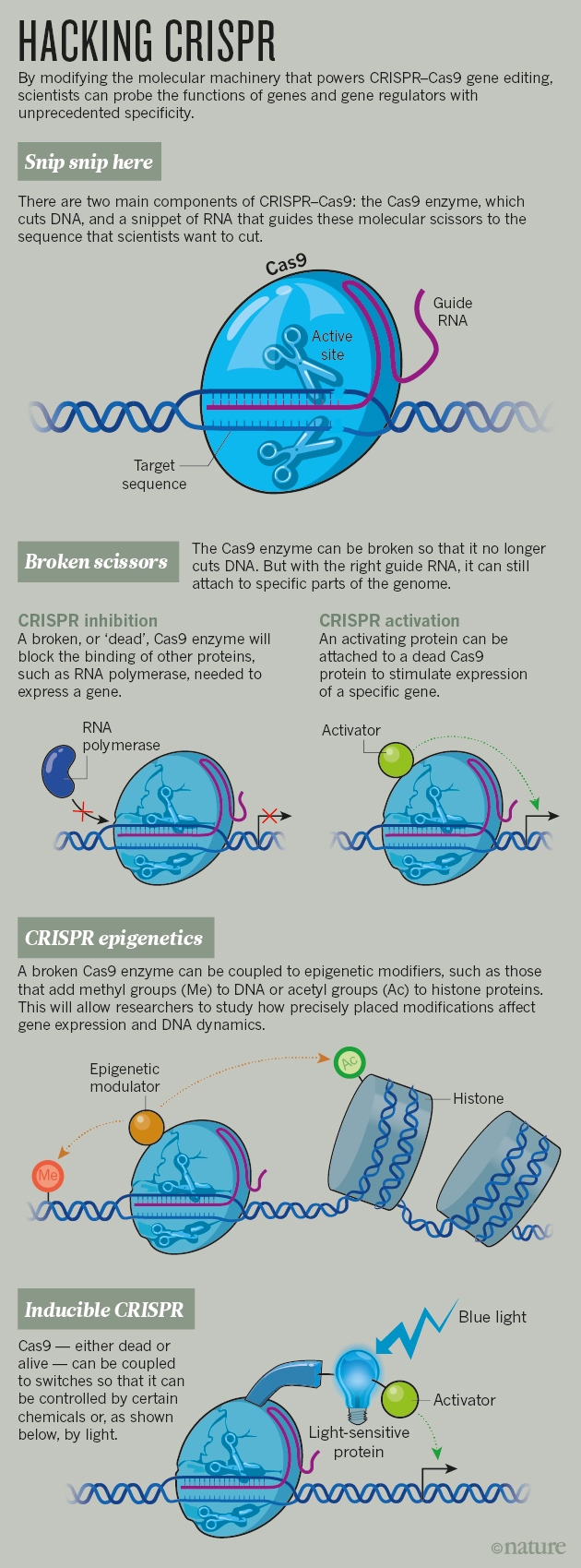
Broken scissors
There are two chief ingredients in the CRISPR–Cas9 system: a Cas9 enzyme that snips through DNA like a pair of molecular scissors, and a small RNA molecule that directs the scissors to a specific sequence of DNA to make the cut. The cell's native DNA repair machinery generally mends the cut — but often makes mistakes.
That alone is a boon to scientists who want to disrupt a gene to learn about what it does. The genetic code is merciless: a minor error introduced during repair can completely alter the sequence of the protein it encodes, or halt its production altogether. As a result, scientists can study what happens to cells or organisms when the protein or gene is hobbled.
But there is also a different repair pathway that sometimes mends the cut according to a DNA template. If researchers provide the template, they can edit the genome with nearly any sequence they desire at nearly any site of their choosing.
In 2012, as laboratories were racing to demonstrate how well these gene-editing tools could cut human DNA, one team decided to take a different approach. “The first thing we did: we broke the scissors,” says Jonathan Weissman, a systems biologist at the University of California, San Francisco (UCSF).
Weissman learned about the approach from Stanley Qi, a synthetic biologist now at Stanford University in California, who mutated the Cas9 enzyme so that it still bound DNA at the site that matched its guide RNA, but no longer sliced it. Instead, the enzyme stalled there and blocked other proteins from transcribing that DNA into RNA. The hacked system allowed them to turn a gene off, but without altering the DNA sequence4.
The team then took its 'dead' Cas9 and tried something new: the researchers tethered it to part of another protein, one that activates gene expression. With a few other tweaks, they had built a way to turn genes on and off at will5.
Several labs have since published variations on this method; many more are racing to harness it for their research6 (see 'Hacking CRISPR'). One popular application is to rapidly generate hundreds of different cell lines, each containing a different guide RNA that targets a particular gene. Martin Kampmann, another systems biologist at UCSF, hopes to screen such cells to learn whether flipping certain genes on or off affects the survival of neurons exposed to toxic protein aggregates — a mechanism that is thought to underlie several neurodegenerative conditions, including Alzheimer's disease. Kampmann had been carrying out a similar screen with RNA interference (RNAi), a technique that also silences genes and can process lots of molecules at once, but which has its drawbacks. “RNAi is a shotgun with well-known off-target effects,” he says. “CRISPR is the scalpel that allows you to be more specific.”
Nik Spencer/Nature
Weissman and his colleagues, including UCSF systems biologist Wendell Lim, further tweaked the method so that it relied on a longer guide RNA, with motifs that bound to different proteins. This allowed them to activate or inhibit genes at three different sites all in one experiment7. Lim thinks that the system can handle up to five operations at once. The limit, he says, may be in how many guide RNAs and proteins can be stuffed into a cell. “Ultimately, it's about payload.”
That combinatorial power has drawn Ron Weiss, a synthetic biologist at the Massachusetts Institute of Technology (MIT) in Cambridge, into the CRISPR–Cas9 frenzy. Weiss and his colleagues have also created multiple gene tweaks in a single experiment8, making it faster and easier to build complicated biological circuits that could, for example, convert a cell's metabolic machinery into a biofuel factory. “The most important goal of synthetic biology is to be able to program complex behaviour via the creation of these sophisticated circuits,” he says.
CRISPR epigenetics
When geneticist Marianne Rots began her career, she wanted to unearth new medical cures. She studied gene therapy, which targets genes mutated in disease. But after a few years, she decided to change tack. “I reasoned that many more diseases are due to disturbed gene-expression profiles, not so much the single genetic mutations I had been focused on,” says Rots, at the University Medical Center Groningen in the Netherlands. The best way to control gene activity, she thought, was to adjust the epigenome, rather than the genome itself.
The epigenome is the constellation of chemical compounds tacked onto DNA and the DNA-packaging proteins called histones. These can govern access to DNA, opening it up or closing it off to the proteins needed for gene expression. The marks change over time: they are added and removed as an organism develops and its environment shifts.
In the past few years, millions of dollars have been poured into cataloguing these epigenetic marks in different human cells, and their patterns have been correlated with everything from brain activity to tumour growth. But without the ability to alter the marks at specific sites, researchers are unable to determine whether they cause biological changes. “The field has met a lot of resistance because we haven't had the kinds of tools that geneticists have had, where they can go in and directly test the function of a gene,” says Jeremy Day, a neuroscientist at the University of Alabama at Birmingham.
CRISPR–Cas9 could turn things around. In April 2015, Charles Gersbach, a bioengineer at Duke University in Durham, North Carolina, and his colleagues published9 a system for adding acetyl groups — one type of epigenetic mark — to histones using the broken scissors to carry enzymes to specific spots in the genome.
The team found that adding acetyl groups to proteins that associate with DNA was enough to send the expression of targeted genes soaring, confirming that the system worked and that, at this location, the epigenetic marks had an effect. When he published the work, Gersbach deposited his enzyme with Addgene so that other research groups could use it — and they quickly did. Gersbach predicts that a wave of upcoming papers will show a synergistic effect when multiple epigenetic markers are manipulated at once.
The tools need to be refined. Dozens of enzymes can create or erase an epigenetic mark on DNA, and not all of them have been amenable to the broken-scissors approach. “It turned out to be harder than a lot of people were expecting,” says Gersbach. “You attach a lot of things to a dead Cas9 and they don't happen to work.” Sometimes it is difficult to work out whether an unexpected result arose because a method did not work well, or because the epigenetic mark simply doesn't matter in that particular cell or environment.
Rots has explored the function of epigenetic marks on cancer-related genes using older editing tools called zinc-finger proteins, and is now adopting CRISPR–Cas9. The new tools have democratized the field, she says, and that has already had a broad impact. People used to say that the correlations were coincidental, Rots says — that if you rewrite the epigenetics it will have no effect on gene expression. “But now that it's not that difficult to test, a lot of people are joining the field.”
CRISPR code cracking
Epigenetic marks on DNA are not the only genomic code that is yet to be broken. More than 98% of the human genome does not code for proteins. But researchers think that a fair chunk of this DNA is doing something important, and they are adopting CRISPR–Cas9 to work out what that is.
Some of it codes for RNA molecules — such as microRNAs and long non-coding RNAs — that are thought to have functions apart from making proteins. Other sequences are 'enhancers' that amplify the expression of the genes under their command. Most of the DNA sequences linked to the risk of common diseases lie in regions of the genome that contain non-coding RNA and enhancers. But before CRISPR–Cas9, it was difficult for researchers to work out what those sequences do. “We didn't have a good way to functionally annotate the non-coding genome,” says Bauer. “Now our experiments are much more sophisticated.”
Farnham and her colleagues are using CRISPR–Cas9 to delete enhancer regions that are found to be mutated in genomic studies of prostate and colon cancer. The results have sometimes surprised her. In one unpublished experiment, her team deleted an enhancer that was thought to be important, yet no gene within one million bases of it changed expression. “How we normally classify the strength of a regulatory element is not corresponding with what happens when you delete that element,” she says.
“I wish I had had this technology sooner. My postdoc would have been a lot shorter.”
More surprises may be in store as researchers harness CRISPR–Cas9 to probe large stretches of regulatory DNA. Groups led by geneticists David Gifford at MIT and Richard Sherwood at the Brigham and Women's Hospital in Boston used the technique to create mutations across a 40,000-letter sequence, and then examined whether each change had an effect on the activity of a nearby gene that made a fluorescent protein10. The result was a map of DNA sequences that enhanced gene expression, including several that had not been predicted on the basis of gene regulatory features such as chromatin modifications.
Delving into this dark matter has its challenges, even with CRISPR–Cas9. The Cas9 enzyme will cut where the guide RNA tells it to, but only if a specific but common DNA sequence is present near the cut site. This poses little difficulty for researchers who want to silence a gene, because the key sequences almost always exist somewhere within it. But for those who want to make very specific changes to short, non-coding RNAs, the options can be limited. “We cannot take just any sequence,” says Reuven Agami, a researcher at the Netherlands Cancer Institute in Amsterdam.
Researchers are scouring the bacterial kingdom for relatives of the Cas9 enzyme that recognize different sequences. Last year, the lab of Feng Zhang, a bioengineer at the Broad Institute of MIT and Harvard in Cambridge, characterized a family of enzymes called Cpf1 that work similarly to Cas9 and could expand sequence options11. But Agami notes that few alternative enzymes found so far work as well as the most popular Cas9. In the future, he hopes to have a whole collection of enzymes that can be targeted to any site in the genome. “We're not there yet,” he says.
CRISPR sees the light
Gersbach's lab is using gene-editing tools as part of an effort to understand cell fate and how to manipulate it: the team hopes one day to grow tissues in a dish for drug screening and cell therapies. But CRISPR–Cas9's effects are permanent, and Gersbach's team needed to turn genes on and off transiently, and in very specific locations in the tissue. “Patterning a blood vessel demands a high degree of control,” he says.
Gersbach and his colleagues took their broken, modified scissors — the Cas9 that could now activate genes — and added proteins that are activated by blue light. The resulting system triggers gene expression when cells are exposed to the light, and stops it when the light is flicked off12. A group led by chemical biologist Moritoshi Sato of the University of Tokyo rigged a similar system13, and also made an active Cas9 that edited the genome only after it was hit with blue light14.
Others have achieved similar ends by combining CRISPR with a chemical switch. Lukas Dow, a cancer geneticist at Weill Cornell Medical College in New York City, wanted to mutate cancer-related genes in adult mice, to reproduce mutations that have been identified in human colorectal cancers. His team engineered a CRISPR–Cas9 system in which a dose of the compound doxycycline activates Cas9, allowing it to cut its targets15.
The tools are another step towards gaining fine control over genome editing. Gersbach's team has not patterned its blood vessels just yet: for now, the researchers are working on making their light-inducible system more efficient. “It's a first-generation tool,” says Gersbach.
Model CRISPR
Cancer researcher Wen Xue spent the first years of his postdoc career making a transgenic mouse that bore a mutation found in some human liver cancers. He slogged away, making the tools necessary for gene targeting, injecting them into embryonic stem cells and then trying to derive mice with the mutation. The cost: a year and US$20,000. “It was the rate-limiting step in studying disease genes,” he says.
A few years later, just as he was about to embark on another transgenic-mouse experiment, his mentor suggested that he give CRISPR–Cas9 a try. This time, Xue just ordered the tools, injected them into single-celled mouse embryos and, a few weeks later — voilá. “We had the mouse in one month,” says Xue. “I wish I had had this technology sooner. My postdoc would have been a lot shorter.”
Researchers who study everything from cancer to neurodegeneration are embracing CRISPR-Cas9 to create animal models of the diseases. It lets them engineer more animals, in more complex ways, and in a wider range of species. Xue, who now runs his own lab at the University of Massachusetts Medical School in Worcester, is systematically sifting through data from tumour genomes, using CRISPR–Cas9 to model the mutations in cells grown in culture and in animals.
Researchers are hoping to mix and match the new CRISPR–Cas9 tools to precisely manipulate the genome and epigenome in animal models. “The real power is going to be the integration of those systems,” says Dow. This may allow scientists to capture and understand some of the complexity of common human diseases.
Take tumours, which can bear dozens of mutations that potentially contribute to cancer development. “They're probably not all important in terms of modelling a tumour,” says Dow. “But it's very clear that you're going to need two or three or four mutations to really model aggressive disease and get closer to modelling human cancer.” Introducing all of those mutations into a mouse the old-fashioned way would have been costly and time-consuming, he adds.
Bioengineer Patrick Hsu started his lab at the Salk Institute for Biological Studies in La Jolla, California, in 2015; he aims to use gene editing to model neurodegenerative conditions such as Alzheimer's disease and Parkinson's disease in cell cultures and marmoset monkeys. That could recapitulate human behaviours and progression of disease more effectively than mouse models, but would have been unthinkably expensive and slow before CRISPR–Cas9.
Even as he designs experiments to genetically engineer his first CRISPR–Cas9 marmosets, Hsu is aware that this approach may be only a stepping stone to the next. “Technologies come and go. You can't get married to one,” he says. “You need to always think about what biological problems need to be solved.”